As a healthcare professional, you're likely all too familiar with the challenges posed by influenza. Every year, the flu season brings with it a host of complications, from mild cases of the sniffles to life-threatening pneumonia. But what if I told you that 2025 is shaping up to be a game-changer in the fight against the flu?
New Frontiers in Influenza Medication
Recent years have seen significant advancements in influenza medication, from the development of new antiviral drugs to innovative approaches to vaccine development. One of the most promising areas of research is in the field of nanotechnology. Scientists are exploring the use of nanoparticles to deliver flu vaccines directly to the lungs, where they can be most effective.
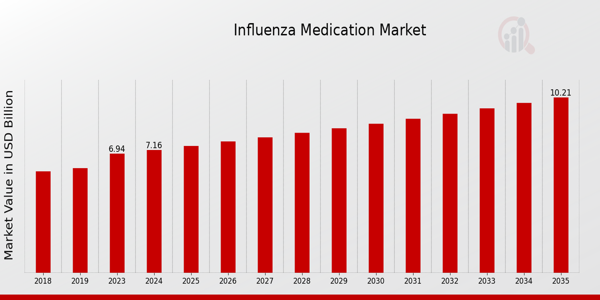
The Influenza Medication Market is projected to grow from USD 7.16 Billion in 2024 to USD 10.2 Billion by 2035, at a CAGR of 3.27%. Increasing seasonal flu outbreaks and advancements in antiviral treatments are key factors driving market expansion.
Read the full market report here: Influenza Medication Market Report.
Baloxavir: A New Antiviral on the Block
Another exciting development is the emergence of baloxavir, a new antiviral medication that has shown promising results in clinical trials. Unlike existing antivirals, which work by inhibiting the replication of the flu virus, baloxavir targets the virus's ability to hijack host cells. This approach has been shown to be effective against a wide range of flu strains, including those that are resistant to existing treatments.
Sustainability and the Future of Influenza Medication
As the world becomes increasingly aware of the need for sustainable healthcare solutions, the development of influenza medication is no exception. Researchers are exploring new approaches to vaccine development that reduce waste and minimize environmental impact. One such approach is the use of cell-based vaccine production, which eliminates the need for eggs in the vaccine production process.
Regulatory Changes: What You Need to Know
Regulatory bodies around the world are taking a closer look at the approval process for influenza medication. In the US, the FDA has introduced new guidelines for the development of antiviral medications, including requirements for more robust clinical trials and improved manufacturing standards. Similar changes are afoot in the EU, where the EMA is introducing new rules for the approval of flu vaccines.
The Role of AI in Influenza Medication
Artificial intelligence (AI) is transforming the field of influenza medication in ways both big and small. From predicting the likelihood of a flu outbreak to identifying potential new targets for antiviral medications, AI is being used to improve our understanding of the flu and develop more effective treatments.
Clinical Applications: Putting AI to Work
So what does the future hold for AI in influenza medication? One potential application is in the development of personalized flu treatments. By analyzing data on an individual's genetic profile, medical history, and lifestyle, AI algorithms can identify the most effective treatment approach for that person. Another area of research is in the use of AI-powered chatbots to help patients manage their flu symptoms and reduce the risk of complications.
Comparison of New Influenza Medications
| Medication | Mechanism of Action | Efficacy | Safety |
|---|---|---|---|
| Baloxavir | Inhibits viral replication | 80-90% effective | Generally well-tolerated |
| Oseltamivir | Inhibits viral neuraminidase | 70-80% effective | Common side effects include nausea, vomiting |
| Zanamivir | Inhibits viral neuraminidase | 70-80% effective | Generally well-tolerated, but may cause bronchospasm |
Key Takeaways
-
Influenza medication is an ever-evolving field, with new developments and advancements emerging all the time.
-
Baloxavir is a new antiviral medication that has shown promising results in clinical trials.
-
Sustainability is becoming an increasingly important consideration in the development of influenza medication.
-
Regulatory changes are afoot, with new guidelines and rules being introduced for the approval of flu vaccines and antiviral medications.
-
AI is being used to improve our understanding of the flu and develop more effective treatments.
Conclusion
As we look to the future of influenza medication, it's clear that there are many exciting developments on the horizon. From new antiviral medications to innovative approaches to vaccine development, researchers are working tirelessly to improve our ability to prevent and treat the flu. Whether you're a healthcare professional, a researcher, or simply someone who's interested in staying up-to-date on the latest developments in the field, I hope this article has provided you with a valuable insight into the future of influenza medication.